
Contact Us


(∆H = +206 kJ/mol): Endothermic reaction
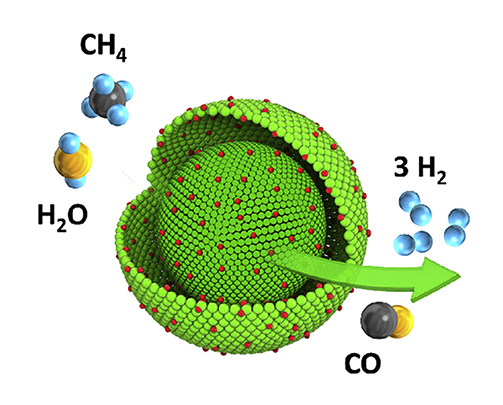

(∆H = -41 kJ/mol): Exothermic reaction
Laboratory : Baek-Un Hall 408, 033-760-2398
Professor office: Baek-Un Hall 309, 033-760-2834
Division of Environmental and Energy Engineering, 1 Yonseidae-gil, Wonju, Gangwon-do, 26493, Republic of Korea